In a pre-release session for the Dec-2020 edition of CAPA Live, Duncan Emerton, PhD, director, custom intelligence at Informa Pharma Intelligence provided a key update on where we currently stand in terms of vaccination programme certification and the path to deployment.
Progress has been unprecedented, but challenges remain. The beginning of vaccinations is an important first step, but it is a long climb before we can effectively manage COVID-19 infections on a global scale. While mass vaccination programmes will likely be commonplace from 2Q 2021 it is estimated that it won't be until the end of 2022 that everyone in the world that wants a vaccine, will get one!
Mr Emerton highlights that in response to the COVID-19 pandemic, the life sciences industry has ramped up its efforts to discover treatments for COVID-19 (the disease) and vaccines against SARSCoV-2. It can take numerous years for vaccines to generally be discovered, tested, trialled and approved for human use, but the industry, regulators and governments have pulled together in this case of emergency to fast-track this process.

Informa Pharma Intelligence's COVID-19/SARS-CoV-2 analytic portal tracks developments in real time and based on data from 01-Dec-2020 shows there are ~3,680 clinical trials and ~950 drugs/vaccines. Of the ~950 drugs/vaccines in development, ~220 are vaccines; of these, ~40 are in clinical development, with the one developed by Pfizer/BioNTech already being deployed in the UK after it received its emergency use authorisation in early Dec 2020.
Other countries are likely to quickly follow approving the Belgian-US collaboration with emergency use authorisation, while the vaccine of American biotechnology company Moderna is also pending its own emergency use authorisation. This will all deliver optimism of a return to some normality, especially in locations where strict mobility restrictions and business closures remain in place, but we need to still be looking medium-term rather than into the immediate future.
By the end of 2020 we will only see 180 million vaccine doses available globally - down from the 780 million initially projected - and vast scaling will be required to capture the general population with initial capacity becoming "rapidly exhausted by front-line and vulnerable " individuals, according to Mr Emerton. "While vaccines may be granted emergency use authorisation within months, demand will heavily outstrip initial capacity," he says.
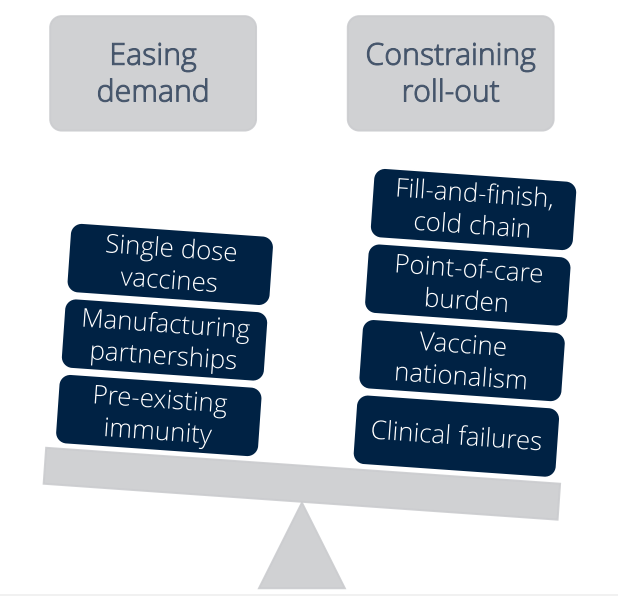
Assuming first vaccines are approved with prime/boost regimen (a requirement for two shots), and taking for a small degree of wastage, 1.8 billion doses will be required to simply meet priority demand. Current forecasts suggest planned supply falls short of potential demand, according to Mr Emerton.
A key challenge remains vaccine distribution, especially as the Pfizer/BioNTech and Moderna examples require ultracold storage throughout the journey from manufacturer to point of care, a process where there are multiple potential points of failure related to temperature and light. Temperature increases from exposure to air or malfunctioning temperature controls can affect vaccine efficacy which once lost cannot be regained, while some vaccines are sensitive to over-cooling and/or light, and if exposed reduces vaccine efficacy, notes Mr Emerton.
CTC - Corporate Travel Community highlighted earlier this year that the potential size of the delivery is enormous. Just providing a single dose to 7.8 billion people would fill 8,000 747 cargo aircraft, according to projections. Land transport will help, especially in developed economies with local manufacturing capacity. But vaccines cannot be delivered globally without the significant use air cargo.
Air cargo already plays a key role in the distribution of vaccines in normal times through well-established time- and temperature-sensitive distribution systems. This capability will be crucial to the quick and efficient transport and distribution of Covid-19 vaccines when they are available, and it will not happen without careful planning, led by governments and supported by industry stakeholders.
On the question of whether or not aviation can do what is needed (not if it has enough capacity), early test flights "seem to have gone well," says Mr Emerton, after all ultracold distribution is a significant new step for the aviation sector.
United Airlines has been testing its supply chain for the Pfizer/BioNTech's vaccine with flights from Brussels to Chicago maintaining the ultracold [−80oC] supply chain, while American Airlines has also started trial flights for Pfizer to "simulate the conditions required for theCOVID-19 vaccine to stress-test the thermal packaging and operational handling process," according to Mr Emerton.
"Quite a lot of unknowns, quite a lot of uncertainties, but there are significant reasons to be cheerful," concludes Mr Emerton.
